走进步长
Company profile
- 联系我们 /tell us
- 地址:西安市高新路50号南洋国际大厦
- 邮编:712000
- 电话:029-88318318(总机)
- 网址:www.buchang.com
四川泸州步长

公司简介(Company Profile)
四川泸州步长生物制药有限公司成立于2014 年9 月17 日,位于四川省泸州高新区医药产业园康乐大道西段480号,注册资金44000 万元。一期投资8.8亿元,建设用地154.7亩,建筑面积7.2万㎡。公司主要经营:生物制品、生物技术、生物药物的研发、生产销售。泸州步长是步长制药在泸州设立的首个也是唯一一个生物制药产业化基地,公司共建设三个原液车间、两个制剂车间及其配套设施。公司作为步长制药生物药物研发产品的产业化生产基地,生产用于治疗肿瘤、骨质疏松、贫血、类风湿关节炎、急性心衰等疾病的抗体、重组蛋白及多肽等药物。 项目全部建成达产后力争成为西南地区最大的生物制品生产基地。不断为国人健康贡献力量。
Sichuan Luzhou Buchang Bio-pharmaceutical Co., Ltd. was established on September 17, 2014, located at No. 480, Kangle Avenue West, Luzhou High-Tech Zone, Sichuan Province, with a registered capital of 440 million yuan. The first phase of investment amounts to 880 million yuan, with a construction land area of 154.7 mu and a building area of 72,000 square meters. The company primarily engages in the R&D, production, and sales of biological products, biotechnology, and biopharmaceuticals. Luzhou Buchang is the first and only biopharmaceutical industrialization base established by Buchang Pharmaceuticals in Luzhou. The company has built three bulk drug workshops, two preparation workshops, and supporting facilities. As the industrialization production base for Buchang Pharmaceuticals' biopharmaceutical R&D products, it produces antibodies, recombinant proteins, and peptides for treating diseases such as tumors, osteoporosis, anemia, rheumatoid arthritis, and acute heart failure. Upon full completion and reaching production capacity, the project aims to become the largest biological product production base in Southwest China, continuously contributing to national health.
原液车间(Bulk Drug Workshops)
公司建成3条符合cGMP要求的原液生产线,分别为1000L规模的原核原液生产线、2*500L规模的真核生产线和2*2000L规模的真核生产线。
1000L规模原核原液生产线: 拥有近2548㎡符合cGMP要求的原核原液生产区域,设计独立的C级种子扩增、D级发酵罐培养间等独立的上、下游生产区域。 上游车间选用先进的生产设备,包括全自动配储液系统及CIP系统、INFORCE(瑞士)恒温摇床、齐志微生物发酵罐、Thermo紫外分光光度计、阿法拉伐连续流碟片离心机、GEA高压均质机、利穗的中控纤维超滤系统、PALL澄清过滤系统等,单批次发酵规模1000L,可满足大肠杆菌、酵母等微生物发酵。下游车间选用先进的生产设备,包括全自动配储液系统及CIP系统,东富龙层析系统、利穗高压层析系统、PALL超滤系统等,可实现3~5部的连续层析及多次超滤纯化工艺实现。
2*500L规模的真核生产线: 拥有近3249㎡符合cGMP要求的真核原液生产区域,设计独立的C级种子扩增、D级生物反应器培养间等独立的上、下游生产区域,下游病毒灭活前和病毒灭活后区域分开。 上游车间选用先进的生产设备,包括奥星的全自动配储液系统及CIP系统、INFORCE(瑞士)二氧化碳培养箱摇床、Applikon100/500L不锈钢生物反应器/Ctyiva200/500L一次性生物反应器、贝克曼全自动细胞计数仪及罗氏Cedex Bio生化分析仪、密理博澄清过滤系统等,单批次发酵规模500L。可满足多品种的上市产品及临床样品的共线生产。下游车间选用先进的生产设备,包括全自动配储液系统及CIP系统,东富龙层析系统、PALL超滤系统等。
2*2000L规模的真核生产线: 拥有近5858㎡符合cGMP要求的真核原液生产区域,车间配置了森松管罐系统并以艾默生的自控系统为基础搭建整体车间的自控系统实现整体车间的联动控制,车间设计独立的C级种子扩增、D级生物反应器培养间等独立的上、下游生产区域,下游病毒灭活前和病毒灭活后区域分开。 上游车间选用先进的生产设备,包括INFORCE(瑞士)二氧化碳培养箱摇床、Ctyiva(美国)的Wave50一次性生物反应器、贝尔芬格(奥地利)100/500L/2000L不锈钢生物反应器、贝克曼全自动细胞计数仪及罗氏Cedex Bio生化分析仪、阿法拉伐2000L/h的全自动碟片式连续流离心机、利穗澄清过滤系统等,单批次培养规模2000L。下游车间选用先进的生产设备,包括Ctyiva 1英寸IC系统、PALL超滤系统等。
The company has established three cGMP-compliant bulk drug production lines:
1000L-scale prokaryotic bulk drug production line: - A 2,548-square-meter cGMP-compliant production area with independent Class C seed expansion, Class D fermentation tank cultivation, and separate upstream/downstream production zones. - The upstream workshop is equipped with advanced production facilities, including a fully automated liquid preparation and storage system, CIP system, INFORCE (Switzerland) constant temperature shaker, Qizhi microbial fermenter, Thermo UV spectrophotometer, Alfa Laval continuous-flow disc centrifuge, GEA high-pressure homogenizer, Lisui's central control fiber ultrafiltration system, and PALL clarification filtration system. With a single-batch fermentation scale of 1000L, the process can meet the requirements for microbial fermentation such as E. coli and yeast. The downstream workshop utilizes advanced production equipment, including a fully automated liquid preparation and storage system, CIP system, Tofflon chromatography system, Lisui high-pressure chromatography system, and PALL ultrafiltration system. These enable 3~5-step continuous chromatography and multiple ultrafiltration purification processes.
2*500L-scale eukaryotic production line: The facility boasts nearly 3,249 square meters of eukaryotic drug substance production area compliant with cGMP requirements, featuring independently designed Class C seed expansion and Class D bioreactor cultivation rooms, as well as separate upstream and downstream production areas, with distinct zones for pre- and post-viral inactivation. The upstream workshop is equipped with advanced production equipment, including Aoxing’s fully automatic liquid preparation and storage system, CIP system, INFORCE (Switzerland) CO₂ incubator shakers, Applikon 100/500L stainless steel bioreactors/Cytiva 200/500L single-use bioreactors, Beckman automated cell counters, Roche Cedex Bio biochemical analyzers, and Millipore clarification filtration systems. The single-batch fermentation capacity reaches 500L, enabling multi-product commercial and clinical sample co-production. The downstream workshop utilizes advanced production equipment, including fully automatic liquid preparation and storage systems, CIP systems, Tofflon chromatography systems, and PALL ultrafiltration systems.
2*2000L-scale eukaryotic production line The facility boasts a cGMP-compliant eukaryotic drug substance production area of approximately 5,858 m². Equipped with Emerson piping and tank system and an Emerson-based automation control system, the workshop achieves integrated control across the entire production line. The design includes dedicated Grade C seed expansion and Grade D bioreactor cultivation rooms, as well as separate upstream and production areas, with distinct zones for pre- and post-virus inactivation. The upstream workshop utilizes advanced production equipment, including: INFORCE (Switzerland) CO₂incubator shakers,Cytiva (USA) Wave50 single-use bioreactors - Balfinger (Austria) 100/500L/2000L stainless steel bioreactors - Beckman automated cell counters and Roche Cedex Bio biochemical analyzers - Alfa Laval 2000L/h fully automated disc-stack continuous-flow centrifuges - Repligen clarification filtration systems The single-batch cultivation capacity reaches 2,000L. The downstream workshop is equipped with advanced production systems, including: - Cytiva 1-inch IC systems - Pall ultrafiltration systems
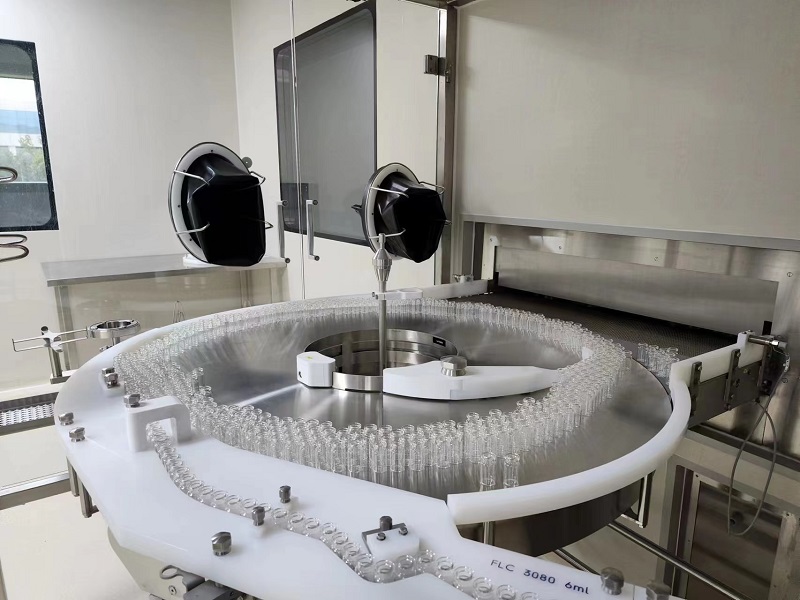
制剂车间(Preparation Workshops)
拥有2条制剂灌装线,采用B+A级实现无菌灌装。洗、烘、灌、轧采用博世的整套灌装联动线。冻干采用东富龙冻干机,可满足2ml~50ml规格的水针、冻干制剂无菌灌装需求。1条制剂线配置500瓶/min的灌装联动线和2*25m²的冻干机,年产能可达1000万瓶;1条制剂线配置350瓶/min的灌装联动线和1*15m²的冻干机,年产能可达500万瓶
The facility is equipped with two formulation filling lines that achieve sterile filling under Grade B+A conditions. The washing, drying, filling, and capping processes are integrated using Bosch’s complete filling line system. Freeze-drying is performed with Tofflon freeze dryers, capable of handling sterile filling for 2ml–50mlliquid and lyophilized formulations. - One filling line is configured with a 500-bottle/min filling system and two 25m² freeze dryers, with an annual capacity of 10 million bottles. - The other filling line is equipped with a 350-bottle/min filling system and one 15m² freeze dryer, with an annual capacity of 5 million bottles. Two sterile filling lines (B/A grade) with Bosch filling systems and Tofflon freeze-dryers, capable of producing water injections and freeze-dried preparations (2ml–50ml). Annual capacities: - Line 1: 500 bottles/min, 10 million bottles/year. - Line 2: 350 bottles/min, 5 million bottles/year.
QC
QC实验室占地1500m²,分别设置有分子生物学实验室、生物学活性实验室、微生物限度室、阳性菌试验室、无菌检测室、免疫学实验室、理化实验室、仪器分析实验室等科室,属于二级生物安全实验室。 QC主要设备均为进口设备,配置Agilent OpenLAB CDS工作站,通过CSV验证,检测质量和数据完整性都得到了有效的保证。
The QC laboratory covers an area of 1,500 square meters and is equipped with various specialized sections, including: - Molecular Biology Laboratory - Biological Activity Laboratory - Microbial Limit Testing Room - Positive Control Laboratory - Sterility Testing Room - Immunology Laboratory - Physicochemical Laboratory - Instrumental Analysis Laboratory It is classified as a Biosafety Level 2 (BSL-2) laboratory. The main QC equipment is all imported, equipped with an Agilent OpenLAB CDS workstation. Through CSV verification, the detection quality and data integrity are effectively guaranteed.
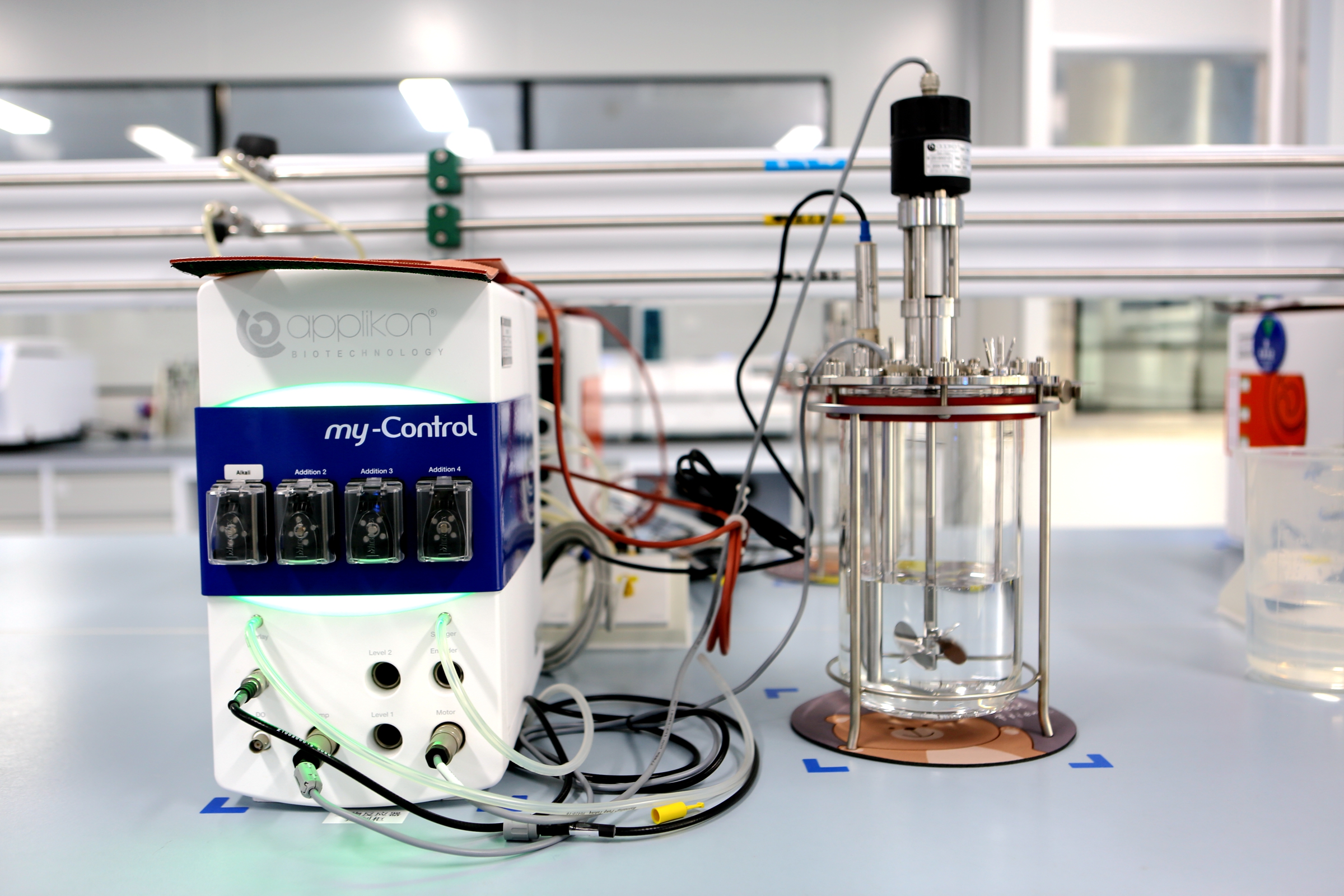
研发实验室简介(Introduction to the R&D Laboratory)
研发实验室专注于生物大分子药物产业化工艺研究与开发的企业实验室,致力于为生物医药领域提供从技术转移到产业化生产的全方位解决方案。 实验室的核心功能包括:技术转移接收确认、基于报产需求的生产工艺表征研究、以及产业化研究。旨在推动生物大分子药物的高效开发与生产。 实验室严格按照GLP(良好实验室规范)标准设计,总投资超过3500万元,于2024年4月正式建成并投入使用。 实验室总面积超过1600㎡,配备了行业领先的研发设备包括:独立纯化水系统、洁净气体系统、8联生物反应器、单联生物反应器、4联发酵罐、单联发酵罐、层析纯化系统、生化分析仪、细胞自动计数仪、超滤系统、HPLC(高效液相色谱仪)、UPLC(超高效液相色谱仪)、QRCR仪(实时荧光定量PCR仪)、酶标仪、电泳仪等。 这些先进的硬件设施使实验室在西南地区处于行业领先地位,具备同时开展5个项目工艺研究的能力,是泸州地区首个具备此类高标准研发能力的实验室。 实验室拥有一支高素质的研发团队,涵盖原核细菌发酵、真核细胞培养、纯化分离、药物质量分析等多个学科领域。团队成员均具备本科以上学历,拥有丰富的研发从业经验,能够为实验室的技术创新和项目推进提供强有力的支持。 自投入使用以来,实验室已成功完成2个项目(EPO、PTH)的纯化工艺表征研究,目前另有5个项目正在同步进行中,为企业的发展注入了持续的动力。未来,实验室将进一步深化与高校、医院及其他药企的合作,推动科研成果的转化与应用。同时,实验室计划逐步承接外部单位的同类研究项目,探索合作共赢的新模式,为生物医药行业的进步贡献力量。 泸州步长研发部实验室将继续秉持创新、严谨、合作的精神,致力于成为生物大分子药物研发与产业化领域的标杆,为全球健康事业提供高质量的解决方案。
The R&D laboratory is an enterprise lab focused on the industrialization process research and development of biomacromolecular drugs, dedicated to providing comprehensive solutions for the biopharmaceutical field, from technology transfer to industrial production. The lab's core functions include: technology transfer acceptance verification, production process characterization studies for regulatory submission requirements, and industrialization research, all aimed at promoting the efficient development and production of biomacromolecular drugs. Designed in strict compliance with GLP (Good Laboratory Practice) standards, the lab represents a total investment of over 35 million yuan and was officially completed and put into operation in April 2024. With a total area exceeding 1,600 square meters, the lab is equipped with industry-leading R&D instruments, including an independent purified water system, a clean gas system, 8-stack bioreactors, single bioreactors, 4-stack fermenters, single fermenters, chromatography purification systems, biochemical analyzers, automated cell counters, ultrafiltration systems, HPLC (High-Performance Liquid Chromatography), UPLC (Ultra-Performance Liquid Chromatography), qPCR instruments (Real-Time Quantitative PCR), microplate readers, and electrophoresis systems. These advanced facilities position the lab as an industry leader in Southwest China, capable of conducting process research for five projects simultaneously. It is the first lab in the Luzhou region with such high-standard R&D capabilities. The lab has a highly skilled R&D team with expertise spanning prokaryotic bacterial fermentation, eukaryotic cell culture, purification and separation, and drug quality analysis. All team members hold at least a bachelor's degree and possess extensive R&D experience, providing strong support for technological innovation and project advancement. Since its launch, the lab has successfully completed purification process characterization studies for two projects (EPO and PTH), with five additional projects currently underway, injecting sustained momentum into the company's growth. In the future, the lab will further strengthen collaborations with universities, hospitals, and other pharmaceutical companies to drive the translation and application of research outcomes. Additionally, the lab plans to gradually undertake similar research projects for external organizations, exploring new models of win-win cooperation to contribute to the advancement of the biopharmaceutical industry. The Luzhou Buchang R&D Laboratory will continue to uphold the principles of innovation, rigor, and collaboration, striving to become a benchmark in the field of biomacromolecular drug R&D and industrialization while delivering high-quality solutions for global health.
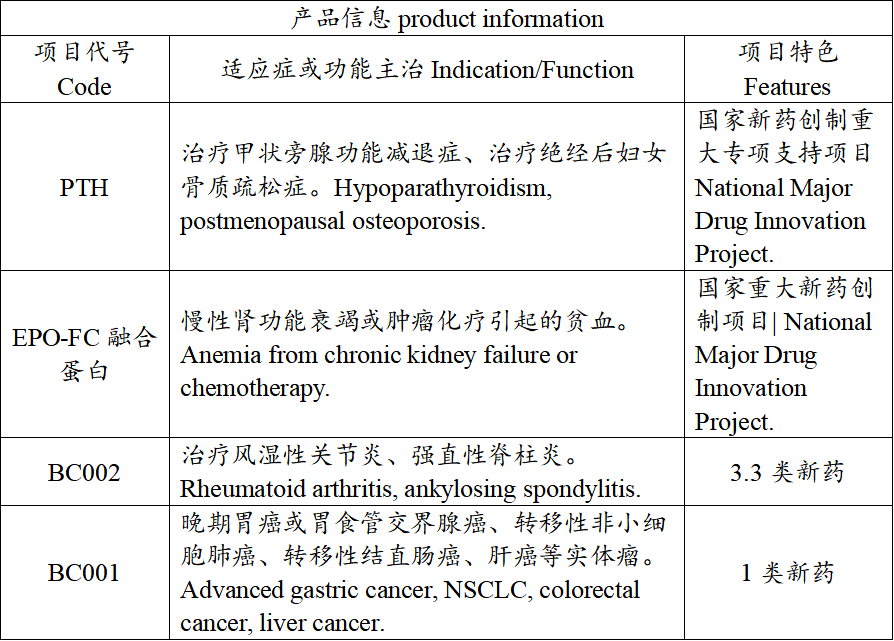
产品信息(product information)
联系方式(Contact Information)
公司邮箱(Company Email):luzhoubuchang@buchangbio.com
质量投诉和药物警戒邮箱(Quality Complaints & Pharmacovigilance Email):luzhoubuchang-qa@buchangbio.com
公司座机电话(Company Landline):+86-0830-6078888
质量投诉电话(Quality Complaints Phone):+86-0830-6078857
药物警戒(Pharmacovigilance Phone):+86-0830-6078879
销售电话(Sales Phone):18608169280